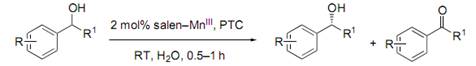来源: | 发布时间:2010-08-13 11:25:00 | 【打印】 【关闭】
羰基合成与选择氧化国家重点实验室清洁催化研究组发展的手性salen-Mn(III)配合物/溴化物体系在水中或水有机两相体系中可温和、高效的实现消旋仲醇的氧化动力学拆分反应;水及溴化物的引入使氧化剂PhI(OAc)2与溴化物形成了一种新的活性氧化剂(Angew. Chem. Int. Ed. 2003, 42, 1042-1044; Chem.-Eur. J. 2005, 11, 1210 – 1216; etc.)。该体系报道后得到了国内外同行的关注与跟踪研究。
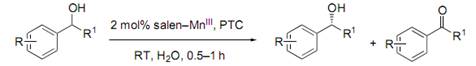
哈佛大学著名化学家 E. J. Corey 教授(1990年诺贝尔化学奖获得者)研究组在最近一期的美国化学会志上发表的研究论文(Mechanism of the Enantioselective Oxidation of Racemic Secondary Alcohols Catalyzed by Chiral Mn(III) Salen Complexes.
J. Am. Chem. Soc. 2010,
132, 11165–11170)中认为:“Chungu Xia and his group showed that chiral Mn(III)-salen complexes can catalyze the oxidation of racemic secondary alcohols of the type R
1R
2CHOH at partial conversion to a mixture of the corresponding ketone and chiral R
1R
2CHOH with excellent enantioselection under the optimized conditions. It was reported that the addition of substoichiometric amounts of a bromide salt to a mixture of catalyst 2a, PhI(OAc)
2, and a biphasic CH
2Cl
2/H
2O medium is important for high enantiose-lectivity and that
krel in favorable cases can be as high as 450.”。Corey研究组对这一体系的反应机理做了进一步的系统研究,阐明了溴离子的作用,认为反应中可能形成了二溴salen-Mn(V)及烷氧基salen-Mn(V)关键中间体。
文章链接:
http://pubs.acs.org/doi/pdfplus/10.1021/ja103103d